History &
Organisation


History
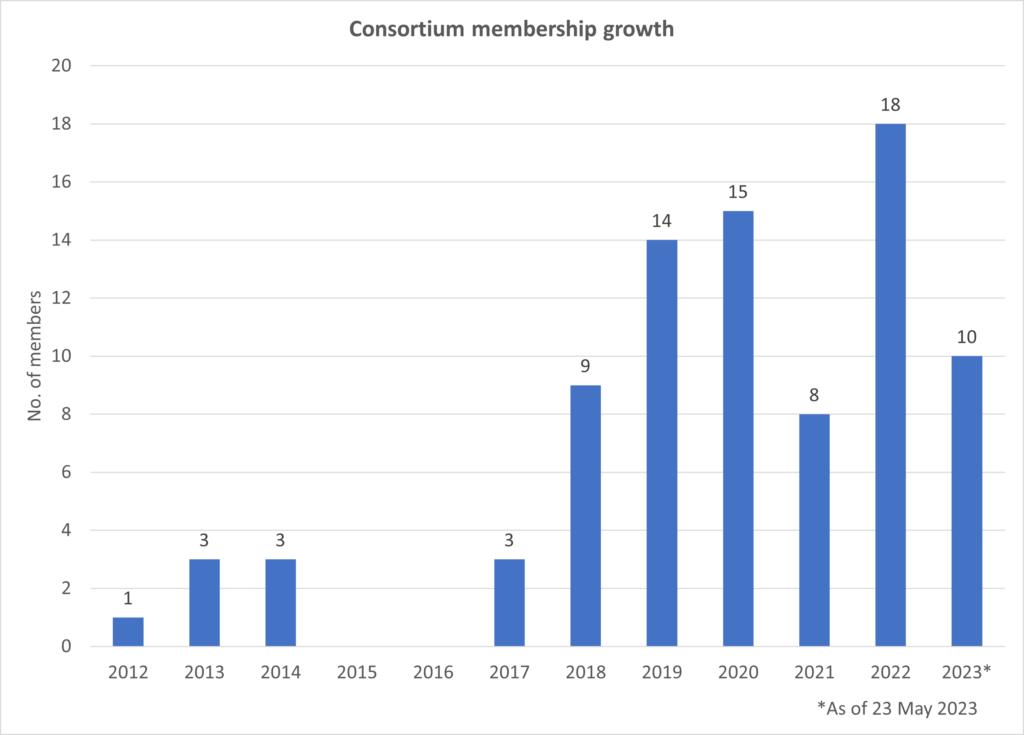
The consortium was established in 2012 by 7 founding members, Elekta and Philips for clinically driven product development and evidence-based introduction of the MR-Linac device.
Today this scientific community of magnetic resonance imaging and radiotherapy experts continues to expand with members across the globe.
Consortium Charter,
Components and Activities
The MR-Linac Consortium (the Consortium) is a collaborative platform intended to facilitate an evidence-based introduction of MRgRT, and to develop and share best practices for an optimized radiation treatment approach to improve patient outcomes.
Achieving this goal is a collaboration between industry and academia. Both parties have a mutual interest in the capabilities of MR-Linac being developed to maximize the clinical impact and the generation of high-quality evidence to demonstrate that impact. Clinical activities and publications within the Consortium are driven by participating sites and individual users. MR-Linac Consortium facilitates this clinical work by developing MR-Linac functionality and to provide a platform in terms of organizational support, technical solutions and appropriate funding.
Consortium Components
Clinical Steering Committee (CSC) – The CSC is the governing body for the main activities and is responsible for the MR-Linac Consortium guidelines.
Tumor Site Groups (TSG) – These are collaborative groups working to establish standard protocols around specific clinical indications, to develop the evidence of clinical value for the specified indication, and to help assure MR-Linac is developed in support of the indications.
Focus Groups (FG) – These are forums with special competence and interest in various technical matters working closely with MR-Linac Consortium product development to propose, evaluate and validate future solutions and functionality. Input to these groups comes, among other things, from TSG.
Brainstorm Groups – The brainstorm groups are informal groups of clinical and technical people who discuss a topic of common interest not covered by other groups.
Consortium Steering Committee (CSC) Role
Clinical Steering – This comprises governance of the Tumor Site Groups, the MOMENTUM project and the Hypothesis Testing Program.
Information Distribution – This pertains to the responsibility for selection and distribution of material related to Consortium activities and made available to the Consortium via the Unity Portal.
The CSC is also responsible for the approval of the final agenda for the annual consortium meeting, the establishment of new Tumor Site groups and for the management of election processes.

Community
The MR-Linac Consortium is a collaborative and welcoming community that keeps growing, with currently over 105 member institutions. We aim to provide a supportive and encouraging peer network where all are welcome to contribute and benefit.
Publications
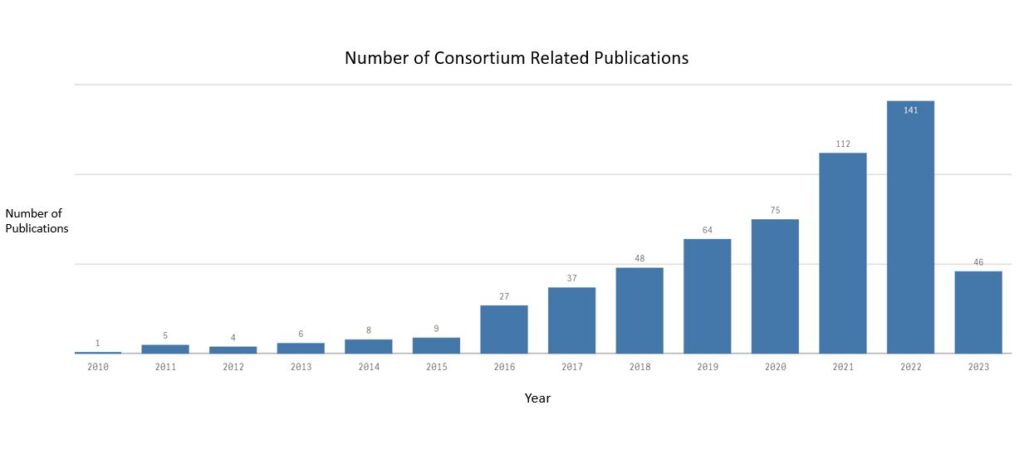
Our members are very proud to have an ever increasing number of publications relating to MRgRT in some of the leading scientific journals as is shown in this graph.